By Kebba Manneh
Gambia National Assembly Select Committee on Health has released its forty-three (43) page report of an inquiry into the death of over 70 Gambian children caused by Acute Kidney Injuries (AKI).
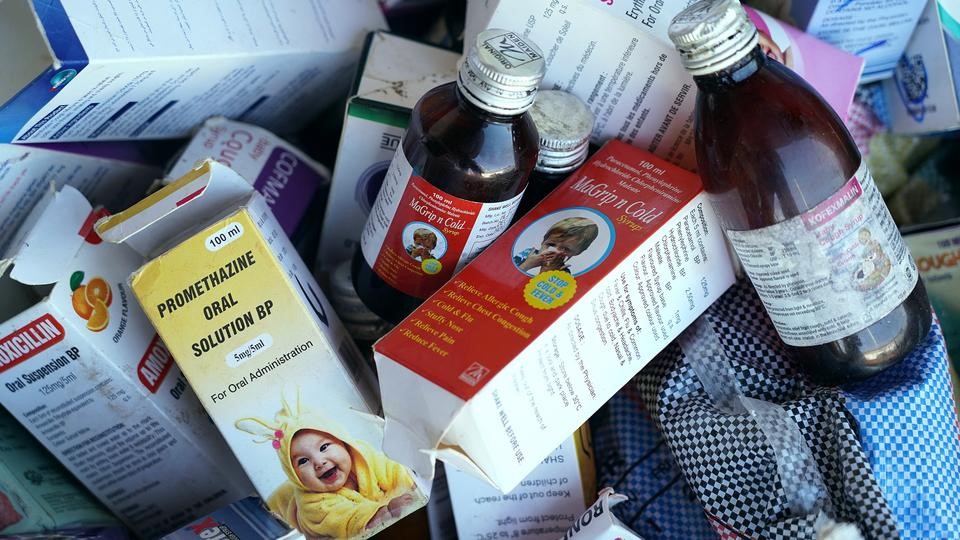
A report released on Tuesday revealed that the death of the children was caused by the consumption of contaminated medical products imported by Atlantic Pharmaceuticals and Manufactured by Maiden Pharmaceutical Ltd India.
The committee recommended that the Gambian Government sue Maiden Pharmaceutical Ltd for the deaths of the children.
“The government should pursue legal action against Maiden Pharmaceuticals for exporting contaminated drugs to The Gambia with the Atlantic brand name. The investigation has revealed that Atlantic pharmacy in The Gambia had followed all regulations for the importation of medicines including the batch that had the contaminated syrups,” Parliamentary inquiry recommended to the government in its report.
It added: “The Select Committee is convinced that Maiden Pharmaceuticals Ltd. is culpable and should be held accountable for exporting the contaminated medicines that were linked to the death of at least 70 children in The Gambia in 2022. The actual cause of death of these children is still under scientific investigation as causality tests are currently being undertaken by the Ministry of Health and partners.”
The Select Committee disclosed that it engaged with two dozen stakeholders and had uncovered fundamental inadequacies in the entire healthcare delivery system of the country, noting that the government of The Gambia through the Ministry of Health should review various recommendations captured in the report and take all necessary measures to address the gaps and challenges within the Health system.
“Findings revealed that all importers of pharmaceutical products (wholesale and retail) into The Gambia are following Medicines Control Agency (MCA) regulations. Notwithstanding, they encounter difficulties with MCA’s operational shortcomings.
“The MCA is confronted with inadequate human and institutional resources,” the Parliamentary Inquiry report revealed.
It added: “The Pharmaceutical Council of The Gambia (PCG) which is responsible for quality control, an inspection of pharmacy premises, and overseeing related regulations is faced with huge logistics and staffing constraints. These need to be addressed to ensure that all residents in The Gambia have access to quality medicines.”
The report further stated that the lack of quality laboratory in the country resulted in the delay in obtaining test results linking the substandard syrups to the AKI, observing that there was an urgent need for a functional National Medicines Quality Control Laboratory (NMQC Lab) in the country to avert future recurrences.
“The Select Committee is reliably informed by the ministry of Health that the World Bank is funding the construction of one modern QCLab.
“The Select Committee, therefore, recommends the government through the Ministry of Health to intensify efforts to complete this task as quickly as possible bearing that it is a requirement under section 50 of the Medicines and Related Product Act 2014,” the Select Committee on Health further indicated in its report.
It added: “The Committee further recommends at this stage to establish the laboratory under the control of MCA as it is the international best practice.”